Dolichodial
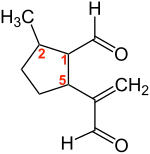 | |
| Names | |
|---|---|
| IUPAC name 2-Methyl-5-(3-oxo-1-propen-2-yl)cyclopentanecarbaldehyde | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| Properties | |
| C10H14O2 | |
| Molar mass | 166.220 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Dolichodial is a natural chemical compound with two aldehyde groups, which belongs to the group of iridoids.
Chemistry
It has in its five-membered ring three asymmetric carbon atoms and accordingly exists in four diastereomeric pairs of enantiomers. The pairs with a different stereochemistry of dolichodial are called anisomorphal and peruphasmal.
-
-(%E2%80%93)-Dolichodial_Stereoisomer_A_V.1.svg.png) (1R,2S,5S)-(–)-Dolichodial (A)
(1R,2S,5S)-(–)-Dolichodial (A) -
-(%252B)-Dolichodial_Stereoisomer_A_V.1.svg.png) (1S,2R,5R)-(+)-Dolichodial (A')
(1S,2R,5R)-(+)-Dolichodial (A') -
-(%252B)-Anisomorphal_Stereoisomer_B_V.1.svg.png) (1S,2S,5S)-(+)-Anisomorphal (B)
(1S,2S,5S)-(+)-Anisomorphal (B) -
-(%E2%80%93)-Anisomorphal_Stereoisomer_B_V.1.svg.png) (1R,2R,5R)-(–)-Anisomorphal (B')
(1R,2R,5R)-(–)-Anisomorphal (B') -
-Peruphasmal_Stereoisomer_C_V.1.svg.png) (1R,2S,5R)-Peruphasmal (C)
(1R,2S,5R)-Peruphasmal (C) -
-Peruphasmal_Stereoisomer_C_V.1.svg.png) (1S,2R,5S)-Peruphasmal (C')
(1S,2R,5S)-Peruphasmal (C') -
-Stereoisomer_D_V.1.svg.png) (1S,2S,5R)-Stereoisomer (D)
(1S,2S,5R)-Stereoisomer (D) -
-Stereoisomer_D_V.1.svg.png) (1R,2R,5S)-Stereoisomer (D')
(1R,2R,5S)-Stereoisomer (D')
Occurrence
Dolichodial and its stereoisomers can be found in the essential oils of certain plants, and also in the defensive secretions of some insect species.[1][2][3]
References
- ^ Tschuch G, Lindemann P, Moritz G (2008). "An unexpected mixture of substances in the defensive secretion of the Tubuliferan thrips, Callococcus fuscipennis". Journal of Chemical Ecology. 34 (6): 742–747. doi:10.1007/s10886-008-9494-3. PMID 18506530. S2CID 19787509.
- ^ Boevé JL, Braekman JC, Daloze D, Houart M, Pasteels JM (1984). "Defensive secretions of Nematinae larvae (Symphyta - Tenthredinidae)". Cellular and Molecular Life Sciences. 40 (6): 546–547. doi:10.1007/BF01982322. hdl:2013/ULB-DIPOT:oai:dipot.ulb.ac.be:2013/81443. S2CID 28094203.
- ^ Dossey AT, Walse S, Edison AS (2008). "Developmental and geographical variation in the chemical defense of the walkingstick insect Anisomorpha buprestoides". Journal of Chemical Ecology. 34 (5): 584–590. doi:10.1007/s10886-008-9457-8. PMID 18401661. S2CID 10765114.